A-3.2 Calculating Air Requirements and Products of Combustion
The efficient and safe operation of gas-fired appliances relies on an adequate supply of air. Later studies will involve sizing and installing the air supply openings and ducts. Understanding the volume of air that a gas-fired appliance requires will help when sizing and installing air supply openings and ducts.
Section A-3.1 The Chemistry of Combustion looked at the products of the combustion process. The quantity of these flue gases will affect the design of the venting systems.
Future studies will involve performing a flue-gas analysis to ensure the safe operation and maximum efficiency of gas appliances. Knowledge of the proper proportions of each product is essential for interpreting the results of the flue-gas analysis.
Air Requirements
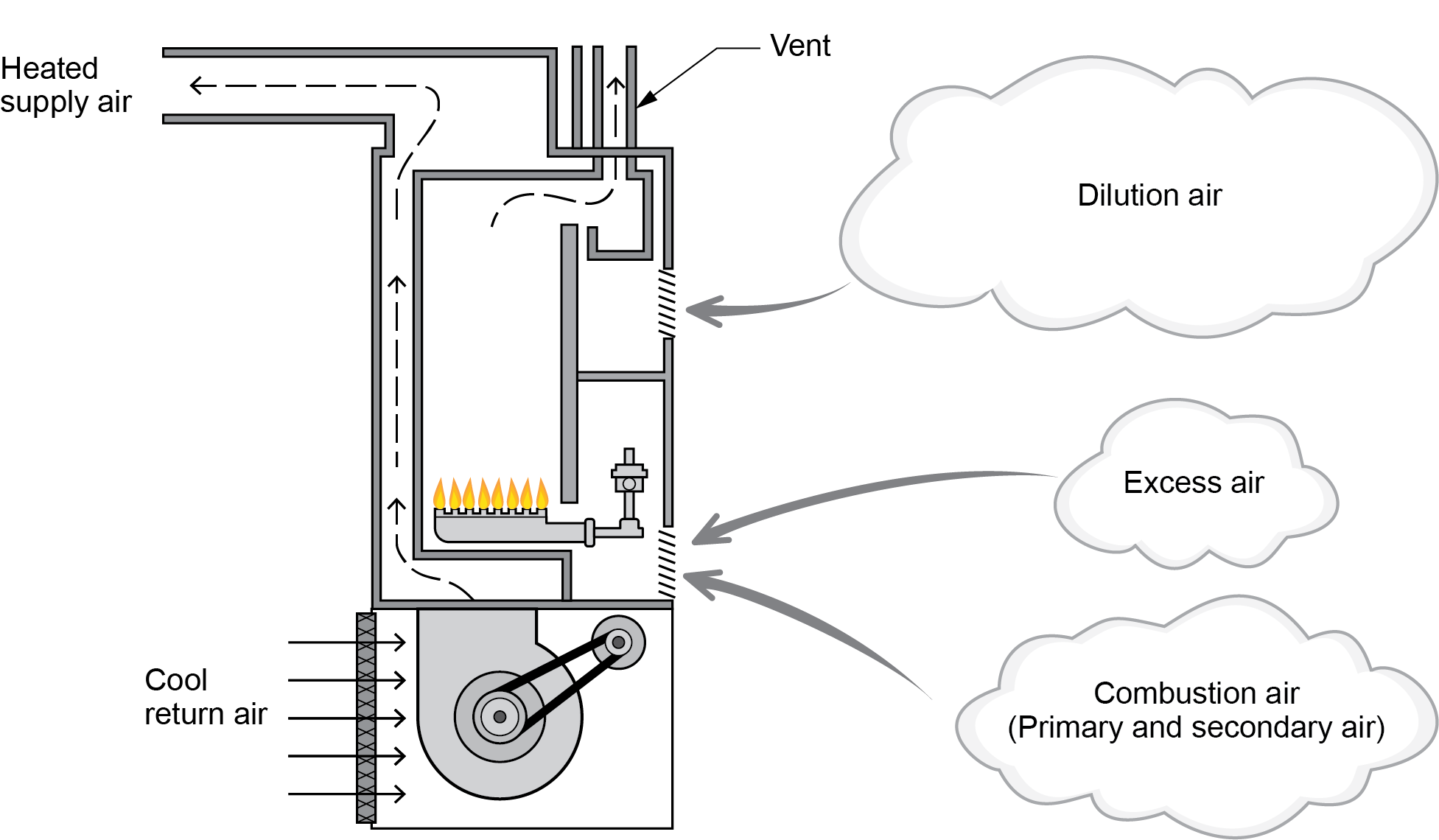
Air supply (Figure 1) has three main categories:
- Combustion air (theoretical)
- Excess air
- Dilution air
Combustion Air
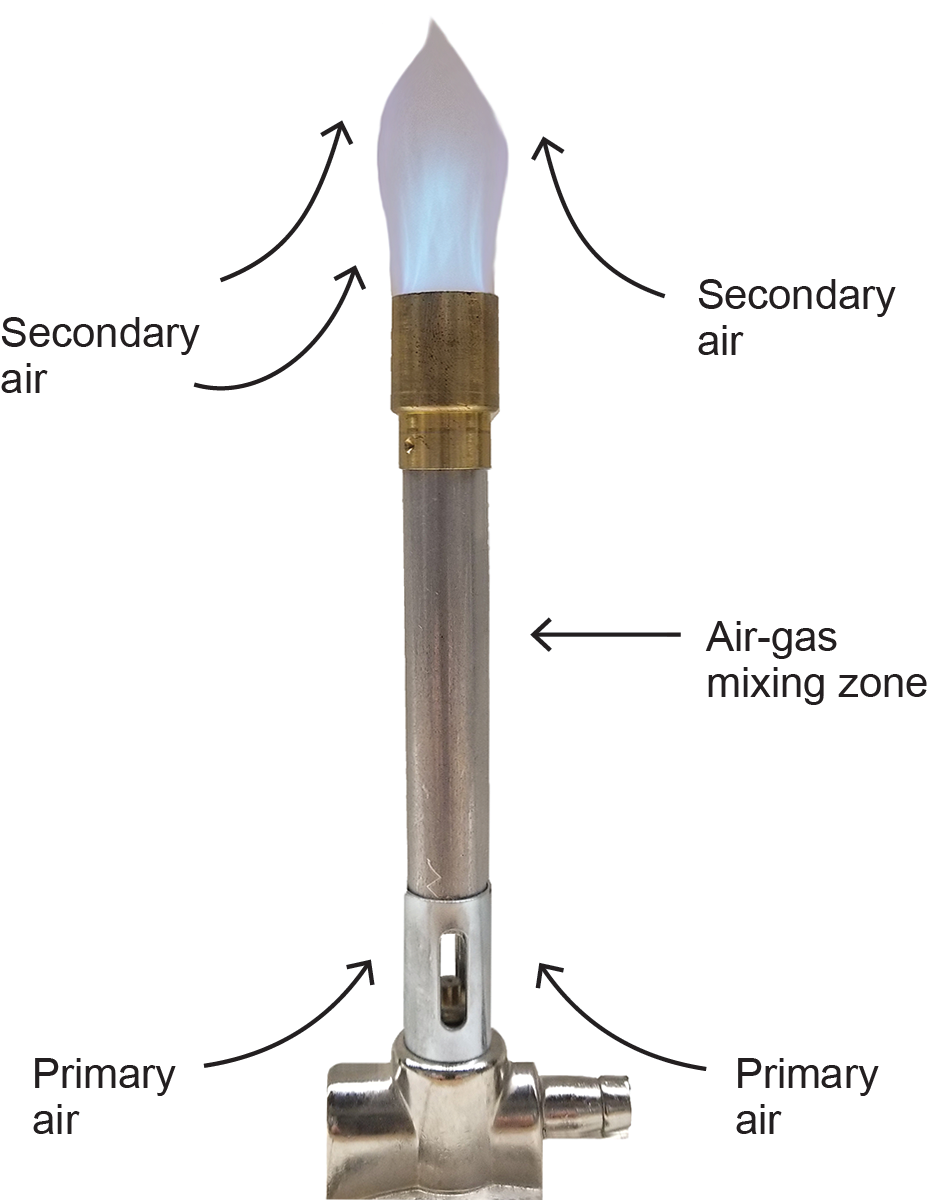
Combustion air (the theoretical amount of air required for the combustion process) is expressed as an air/gas ratio and is 10:1 for natural gas and 25:1 for propane. Combustion air can be further subdivided into two classifications: primary and secondary air. The combustion air mixed with the fuel gas before the ignition point is referred to as “primary air” (Figure 2). For a typical non-mechanical burner, the amount of primary air used is normally considered to be one-third of the combustion air. Depending on the mixing capability of a mechanical burner, the primary air ratio will be much higher.
The secondary air is required to complete the combustion process and is supplied at the flame. Secondary air is generally considered to be the other two-thirds of the combustion air required for perfect combustion (Figure 2).
Excess Air
To ensure that all fuel is completely burned, excess air must be introduced, often half as much again as would be needed for stoichiometric combustion.
When extra air is introduced, more gases must be heated by the same amount of BTU’s. This decreases the initial temperature of the flue gas. At the same time, a larger volume of flue gas is forced through the heat exchanger in a shorter period of time, causing a rapid fall in heating efficiency. Therefore, enough excess air must be made available to ensure complete combustion without drastically reducing the appliance’s efficiency.
Compared to the combustion air supply, most mechanical burners can achieve complete combustion with as little as 20% to 30% excess air, while some atmospheric burners require as much as 50% excess air.
Considering worst-case scenarios, the amount of excess air that must be available to a gas-fired appliance is another 50% of that required for perfect combustion.
Dilution Air
Natural draft appliances are equipped with a draft control device that draws additional ambient air into the venting system to control the draft influence on the combustion chamber. This air also cools the hot vent gases prevalent in these lower-efficiency appliances.
If an appliance has a draft control device, such as a draft hood, draft diverter, or barometric damper, the required volume of dilution air usually equals the total of the combustion and excess air supplied to the burner.
Modern high-efficiency appliances have much lower flue-gas temperatures and, therefore, will not have a draft control device because they do not require dilution air.
Air Supply Volumes
The chemical formula for perfect combustion was examined in Section 3.1 The Chemistry of Combustion. There, in terms of volume, it described every unit of natural gas as combining with 10 units of air and every unit of propane requiring 25 units of combustion air. The other air volumes needed were then given as percentages of the theoretical combustion air requirements. Therefore, the total air requirements can be calculated based on the appliance input and type of gas. The calculations for the different categories of air supply are shown below for natural and propane gas.
Air Requirements for Natural Gas
[latex]\color{blue}{\dfrac{\text{Input}}{\text{Caloric Value}}=\text{ Gas Flow Rate}}[/latex]
[latex]\text{Combustion Air}=\text{Gas Flow Rate}\times10\,(10:1)[/latex]
- Primary Air = [latex]\tfrac{1}{3}[/latex] of Combustion Air
- Secondary Air = [latex]\tfrac{2}{3}[/latex] of Combustion Air
[latex]\text{Excess Air}=\text{Gas Flow Rate}\times5\,(50\%\text{ of Combustion Air,}\,5:1)[/latex]
[latex]\text{Dilution Air}=\text{Gas Flow Rate}\times15\,(\text{Combustion Air}+\text{Excess Air,}\,15:1)[/latex]
[latex]\text{Total Air}=\text{Gas Flow Rate}\times30\,(\text{Combustion Air}+\text{Excess Air}+\text{Dilution Air,}\,30:1)[/latex]
Air Requirements for Propane Gas
[latex]\color{blue}{\dfrac{\text{Input}}{\text{Caloric Value}}=\text{ Gas Flow Rate}}[/latex]
[latex]\text{Combustion Air}=\text{Gas Flow Rate}\times25\,(25:1)[/latex]
- Primary Air = [latex]\tfrac{1}{3}[/latex] of Combustion Air
- Secondary Air = [latex]\tfrac{2}{3}[/latex]of Combustion Air
[latex]\text{Excess Air}=\text{Gas Flow Rate}\times12.5\,(50\%\text{ of Combustion Air,}\,12.5:1)[/latex]
[latex]\text{Dilution Air}=\text{Gas Flow Rate}\times37.5\,(\text{Combustion Air}+\text{Excess Air,}\,37.5:1)[/latex]
[latex]\text{Total Air}=\text{Gas Flow Rate}\times75\,(\text{Combustion Air}+\text{Excess Air}+\text{Dilution Air,}\,75:1)[/latex]
In reality, the combustion air supply requirements should be the same, whether the appliance operating on propane or natural gas (10 ft3 of air for each 1,000 BTU/h of input). Because a 100,000 BTU/h appliance requires a gas flow rate of 100 CFH when fired on natural gas but only 40 CFH on propane, it maintains the air to gas ratios.
Calculating the Volume of Combustion Gases
As discussed in the previous section, the products of complete combustion are carbon dioxide (CO2), water vapour (H2O), and nitrogen (N2). The composition of these flue gases depends on the type of gas and the air-gas ratio.
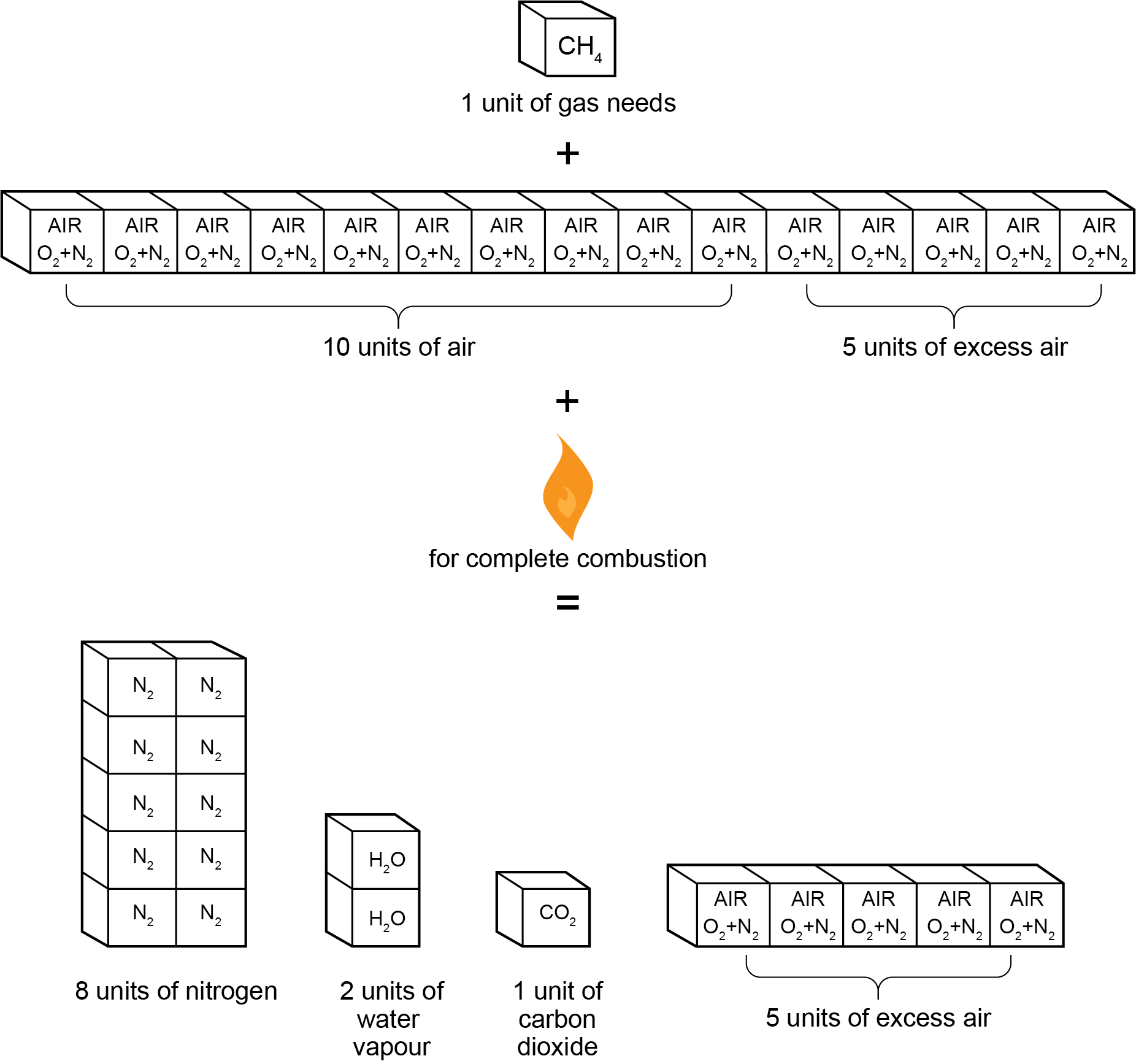
The following is the complete combustion formula for natural gas with excess air:
[latex]\color{blue}{\text{CH}_4+2\text{O}_2+8\text{N}_2+\text{Excess Air}\rightarrow\text{CO}_2+2\text{H}_2\text{O}+8\text{N}_2+\text{Excess Air}+\text{Heat}}[/latex]
Figure 3 shows that every unit of natural gas consumed produces one unit of carbon dioxide, two units of water vapour, and eight units of nitrogen. The amount of excess air found in the flue gas equals the exact amount of excess air introduced prior to combustion.
The following is the complete combustion formula for propane gas with excess air:
[latex]\color{blue}{\text{C}_3\text{H}_8+5\text{O}_2+20\text{N}_2+\text{Excess Air}\rightarrow3\text{CO}_2+4\text{H}_2\text{O}+20\text{N}_2+\text{Excess Air}+\text{Heat}}[/latex]
In terms of volume, every unit of propane gas consumed produces three units of carbon dioxide, four units of water vapour, and 20 units of nitrogen. Just as with the natural gas, the amount of excess air found in the flue gas equals the exact amount of excess air introduced prior to combustion.
Do not conclude from this equation that a given appliance operating on propane instead of natural gas would have 2.5 times as much flue gas. As was mentioned previously, the appliance would only require 40% as much initial propane fuel for the same heat energy input; therefore, the result would be the same volume of flue gas.
The calculated quantities of the different products of combustion based on the appliance input and type of gas are below.
Products of Combustion for Natural Gas
[latex]\color{blue}{\dfrac{\text{Input}}{\text{Caloric Value}}=\text{ Gas Flow Rate}}[/latex]
[latex]\text{Gas Flow Rate}\times1=\text{Carbon Dioxide}[/latex]
[latex]\text{Gas Flow Rate}\times2=\text{Water Vapour}[/latex]
[latex]\text{Gas Flow Rate}\times8=\text{Nitrogen}[/latex]
Products of Combustion for Propane Gas
[latex]\color{blue}{\dfrac{\text{Input}}{\text{Caloric Value}}=\text{ Gas Flow Rate}}[/latex]
[latex]\text{Gas Flow Rate}\times3=\text{Carbon Dioxide}[/latex]
[latex]\text{Gas Flow Rate}\times4=\text{Water Vapour}[/latex]
[latex]\text{Gas Flow Rate}\times20=\text{Nitrogen}[/latex]
In further studies, evaluations of combustion efficiency will be made by analyzing the actual composition of the flue-gas products compared to the theoretical values.
The following is a sample calculation of both the air supply and products of combustion for a 100,000 BTU/h natural gas boiler with a draft control operating at 50% excess air:
Gas Flow Rate
[latex]\color{blue}{\dfrac{(100\,000\text{ Btuh})}{(1\,000\text{ Btu/ft}^3)}=100\text{ CFH}}[/latex]
Total Air Supply Calculation
Combustion Air:
[latex]\color{blue}{100\text{ CFH}\times10=1\,000\text{ CFH}}[/latex]
- [latex]\tfrac{1}{3}[/latex] Primary Air = 333.333 CFH
- [latex]\tfrac{2}{3}[/latex] Secondary Air = 666.667 CFH
Excess Air ([latex]\text{Combustion Air}\times50\%[/latex]):
[latex]\begin{array}{ccc}\color{blue}{100\text{ CFH}\times5=500\text{ CFH}}&\text{or}&\color{blue}{1\,000\text{ CFH}\times0.5=500\text{ CFH}}\end{array}[/latex]
Dilution Air ([latex]\text{Combustion Air}+\text{Excess Air}[/latex]):
[latex]\begin{array}{ccc}\color{blue}{100\text{ CFH}\times15=1\,500\text{ CFH}}&\text{or}&\color{blue}{1\,000\text{ CFH}+500\text{ CFH}=1\,500\text{ CFH}}\end{array}[/latex]
Products of Combustion Calculation
[latex]\color{blue}{\text{CO}_2\text{: }100\text{ CFH}\times1=100\text{ CFH}}[/latex]
[latex]\color{blue}{\text{H}_2: 100\text{ CFH}\times2=200\text{ CFH}}[/latex]
[latex]\color{blue}{\text{N}_2: 100\text{ CFH}\times8=800\text{ CFH}}[/latex]
 Self-Test A-3.2: Calculating Air Requirements and Products of Combustion
Self-Test A-3.2: Calculating Air Requirements and Products of Combustion
Complete Self-Test A-3.2 and check your answers.
If you are using a printed copy, please find Self-Test A-3.2 and Answer Key at the end of this section. If you prefer, you can scan the QR code with your digital device to go directly to the interactive Self-Test.

References
Skilled Trades BC. (2021). Book 1: Fuel gas systems, Heating and cooling systems. Plumber apprenticeship program level 2 book 1 (Harmonized). Crown Publications: King’s Printer for British Columbia.
Trades Training BC. (2021). A-3: Air supply for gas appliances. In: Plumber Apprenticeship Program: Level 2. Industry Training Authority, BC.
Media Attributions
All figures are used with permission from Skilled Trades BC (2021) unless otherwise noted.
The air required for the combustion process, expressed as an air/gas ratio (10:1 for natural gas, 25:1 for propane). (Section A-3.2)
The portion of combustion air mixed with the fuel gas before ignition, typically one-third of the total combustion air. (Figure 2, Section A-3.2)
The additional air required to complete the combustion process, typically two-thirds of the total combustion air. (Section A-3.2)
Extra air supplied to the combustion process beyond the amount required for perfect combustion, typically 20-30% more than the theoretical amount needed for stoichiometric combustion. This ensures that all fuel is burned efficiently and completely. (Section A-3.1 and Section A-3.2)
A unit of energy used to measure the heat content of fuel. (Section A-3.2)
The ideal burning process where there is enough oxygen to burn all the fuel completely, producing only carbon dioxide (CO₂), water vapor (H₂O), and heat as byproducts. (Section A-3.1)
Ambient air introduced into the venting system of natural draft appliances, used to control draft and cool vent gases. (Section A-3.2)
The amount of gas flowing through an appliance, typically measured in cubic feet per hour (CFH), and used to calculate air supply requirements. (Section A-3.2)

