A-3.1 The Chemistry of Combustion
Combustion is the rapid oxidation of fuel accompanied by the production of heat or heat and light.
Requirements for Combustion
Starting and sustaining combustion requires three ingredients mixed together in the correct proportions and one reaction:
- Fuel: most fuels are a chemical structure of carbon and hydrogen and, for that reason, are called hydrocarbons. In the gasfitting trade, these are normally natural gas, propane, or butane, which are burned in a gaseous state.
- Heat: enough heat must be supplied to bring the fuel up to its ignition temperature. This initial heat can be supplied by a pilot flame, spark ignition, or hot surface igniter. Ignition temperatures vary according to the type of gas used:
-
- Natural gas, approximately 1,300°F; 700°C
- Propane, 920°F; 495°C
- Butane 900°F; 480°C
- Oxygen: the combustion process consumes oxygen, which is obtained from the surrounding air or by a combustion fan or blower. Because air contains only 20% oxygen and 80% nitrogen, the process requires a much larger volume of air than it would require if using pure oxygen.
Combustion is a chemical chain reaction that creates heat, some of which will provide enough energy to make the reaction self-sustaining (Figure 1).
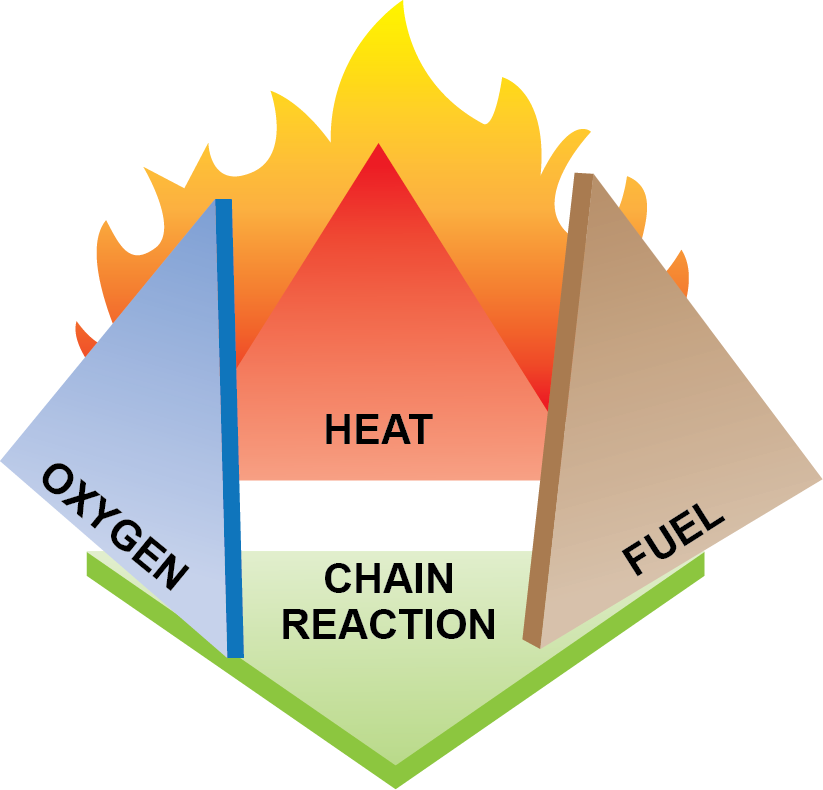
If any one of the three elements are absent, combustion will not take place nor will it support itself after an element is removed.
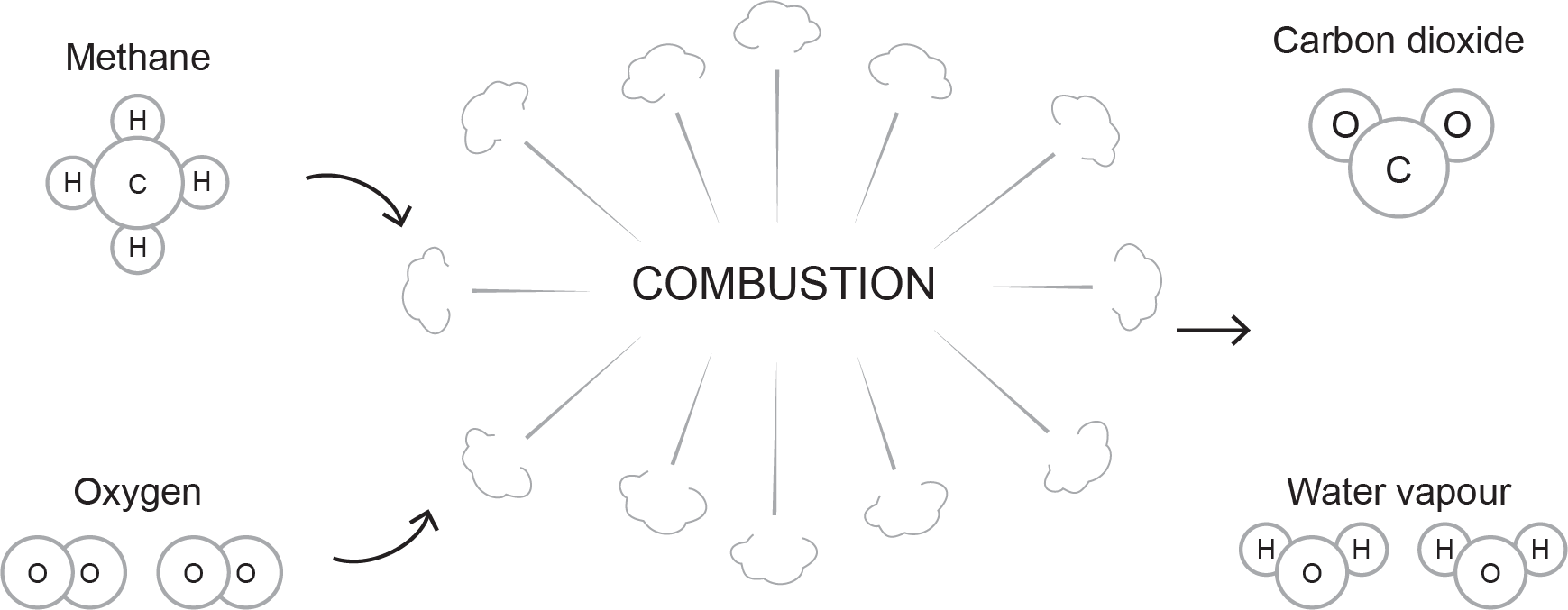
The chemistry of combustion shows the reaction that takes place between fuel gas and oxygen when heated to ignition temperature. Using hydrocarbon fuels with enough oxygen to support combustion produces carbon dioxide (CO2), water vapour (H2O), and heat.
The type of hydrocarbon fuel used will determine the amount of oxygen required as well as the quantity of CO2 and H2O the reaction will yield. The chemical formula for the combustion reaction of natural gas and oxygen is:
[latex]\color{blue}{\text{CH}_4+2\text{O}_2\rightarrow\text{CO}_2+2\text{H}_2\text{O}+\text{Heat}}[/latex]
Figure 2 illustrates the chemical combustion of natural gas (methane) and oxygen.
The chemical formula expressing the combustion reaction for propane and oxygen is:
[latex]\color{blue}{\text{C}_3\text{H}_8 +5\text{O}_2\rightarrow3\text{CO}_2+4\text{H}_2\text{O}+\text{Heat}}[/latex]
Notice that the yield of CO2 and H2O creates a balanced formula driven by the number of individual carbon and hydrogen atoms in the hydrocarbon fuel compound. This determines the number of O2 elements required.
For gas appliances, the source of oxygen is air, which has approximately 20% oxygen, 79% nitrogen, and 1% of other gases. This section describes three types of combustion, each determined by the initial air supply:
- Perfect combustion
- Incomplete combustion
- Complete combustion
Perfect (Stoichiometric) Combustion
Perfect (stoichiometric) combustion refers to the theoretical (or mathematically exact) volume of air that must be mixed with fuel gas to achieve perfect combustion. The formula for perfect combustion is used for theoretical calculations only. This is because most gas burners are not capable of mixing mathematically exact gas and air volumes together to produce complete combustion.
As previously stated, air is composed of approximately 20% oxygen (O2) and 80% nitrogen (N2).
Therefore, the perfect combustion formula for natural gas is expressed as follows:
[latex]\color{blue}{\text{CH}_4+2\text{O}_2+8\text{N}_2\rightarrow\text{CO}_2+2\text{H}_2\text{O}+8\text{N}_2+\text{Heat}}[/latex]
Notice that the ratio of oxygen required for each unit of natural gas is still 2:1, but now that air is being used, for every one unit of oxygen comes four units of nitrogen.
Figure 3 illustrates the formula.
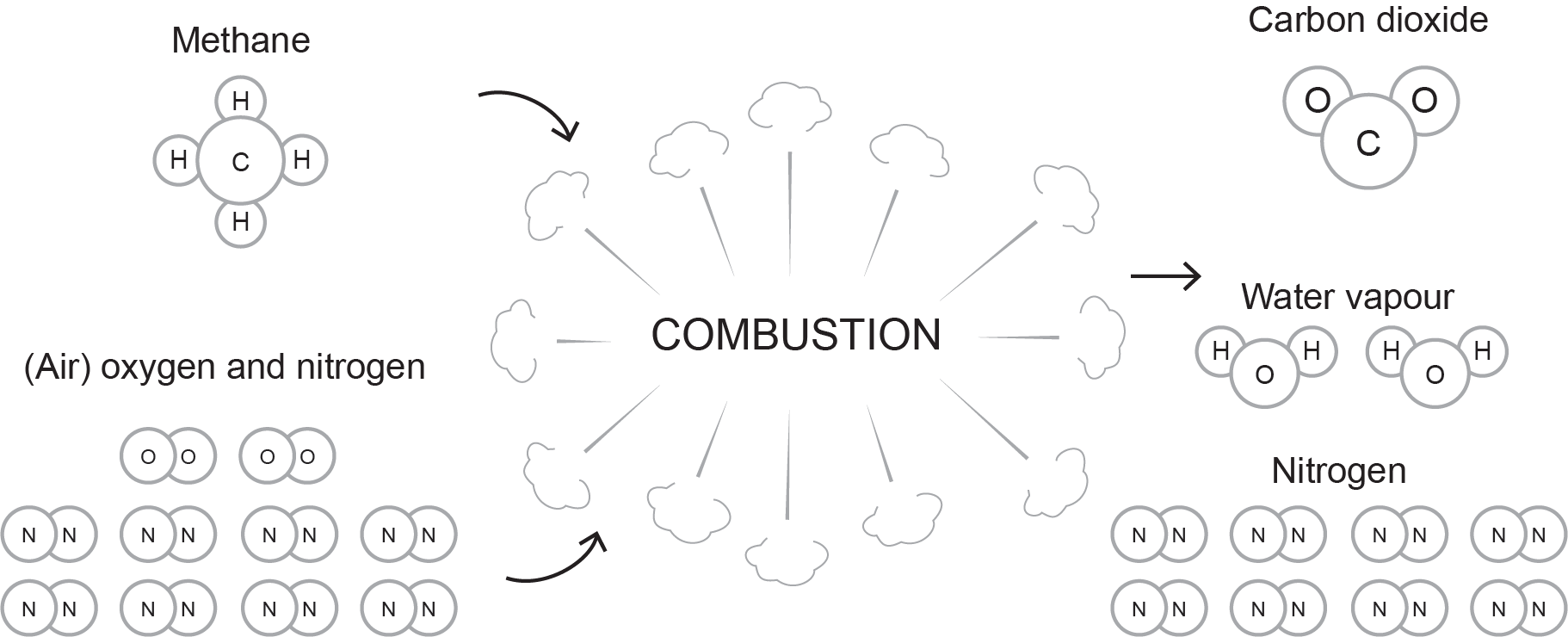
Notice that, compared to Figure 2, we have now introduced nitrogen into the combustion process. Nitrogen is an inert gas that remains stable and will not chemically react, provided that the temperatures are not too high. Nitrogen does increase the total volume of flue gas, thereby absorbing some of the heat produced by the reaction. At high temperatures, some nitrogen will bind to oxygen to form nitric oxide and nitrogen dioxide, commonly referred to as NOx gases. Burner design can affect NOx production
As mentioned earlier, the number of individual carbon and hydrogen atoms in the hydrocarbon fuel compound determines the number of O2 elements required. Therefore, propane would yield the following perfect combustion formula, requiring 25 units of air for every one unit of C3H8.
[latex]\color{blue}{\text{C}_3\text{H}_8+5\text{O}_2+20\text{N}_2\rightarrow3\text{CO}_2+4\text{H}_2\text{O}+20\text{N}_2+\text{Heat}}[/latex]
Incomplete Combustion
Where there is not enough oxygen to burn the fuel gas completely, incomplete combustion occurs, leaving unburned fuel in the flue gas. Over-firing a burner can also cause incomplete combustion because it affects gas/air mixtures. Depending on the degree of incomplete combustion, the flue gas will contain varying amounts of the following products of incomplete combustion:
- Carbon (C)
- Hydrogen (H2)
- Carbon monoxide (CO)
- Complex chains of alcohols called aldehydes
Carbon is easily observed because it forms a black powdery substance known as soot. Soot is an immediate visible indicator of incomplete combustion. However, the absence of visible soot does not necessarily indicate complete combustion.
Free hydrogen in the combustion process indicates that there is a high level of incomplete combustion. Normally, H2 is not present unless the CO level is very high.
Carbon monoxide is a toxic, odourless, tasteless gas that, if inhaled in even small quantities, may cause death. Because it is one of the first products of incomplete combustion, it may be present before any of the other products are created.
Aldehydes are very toxic and poisonous. Because they are acrid in odour and irritate the eyes, nose, and throat, they are easily detected. Whenever aldehydes form, it is quite likely that CO is also present in the flue gas, but CO can be present without aldehydes.
Carbon monoxide and aldehydes in any quantity are toxic and life threatening.
The following formula and accompanying Figure 4 express the incomplete combustion of natural gas when only half of the theoretical air for perfect combustion was supplied.
[latex]\color{blue}{\text{CH}_4+1\text{O}_2+4\text{N}_2\rightarrow\text{CO}+\text{H}_2\text{O}+\text{H}_2+4\text{N}_2+\text{Heat}}[/latex]
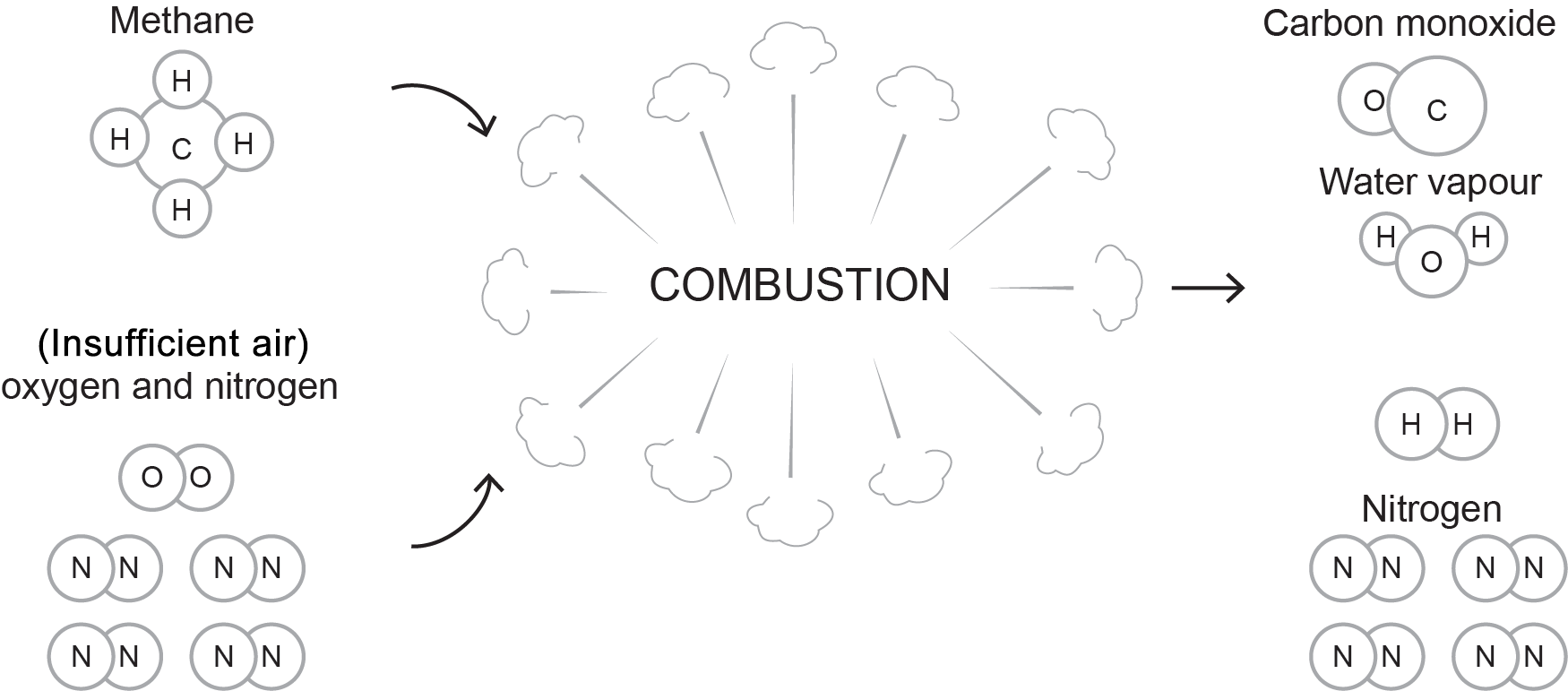
It is important to remember that incomplete combustion occurs not only from a lack of oxygen. Temperature, time, and fuel-air mixing also affect the combustion process.
If the flame is cooled to or just below its ignition temperature, incomplete combustion will occur. Conditions that could cool the flame include the flame impinging on a cold metal surface, such as a heat exchanger or boiler refractory, or a high draft through the combustion chamber, possibly caused by a cracked heat exchanger.
A dirty or poorly designed burner that does not allow for proper gas/air mixing can also produce higher levels of carbon monoxide and aldehyde.
Complete Combustion
If the burner is supplied with only the exact amount of theoretical air required, some of the carbon and hydrogen atoms would not be united with enough oxygen in the short period of combustion time and, therefore, incomplete combustion would occur.
To achieve complete combustion, the combustion process must have more air than perfect combustion would require. This excess air supply ensures that all atoms of carbon and hydrogen unite with enough atoms of oxygen to complete the combustion process.
The complete combustion formula for natural gas is expressed as:
[latex]\color{blue}{\text{CH}_4+2\text{O}_2+8\text{N}_2+\text{Excess Air}\rightarrow\text{CO}_2+2\text{H}_2\text{O}+8\text{N}_2+\text{Excess Air}+\text{Heat}}[/latex]
Figure 5 illustrates this formula when using 50% more air than required for perfect combustion (excess air).
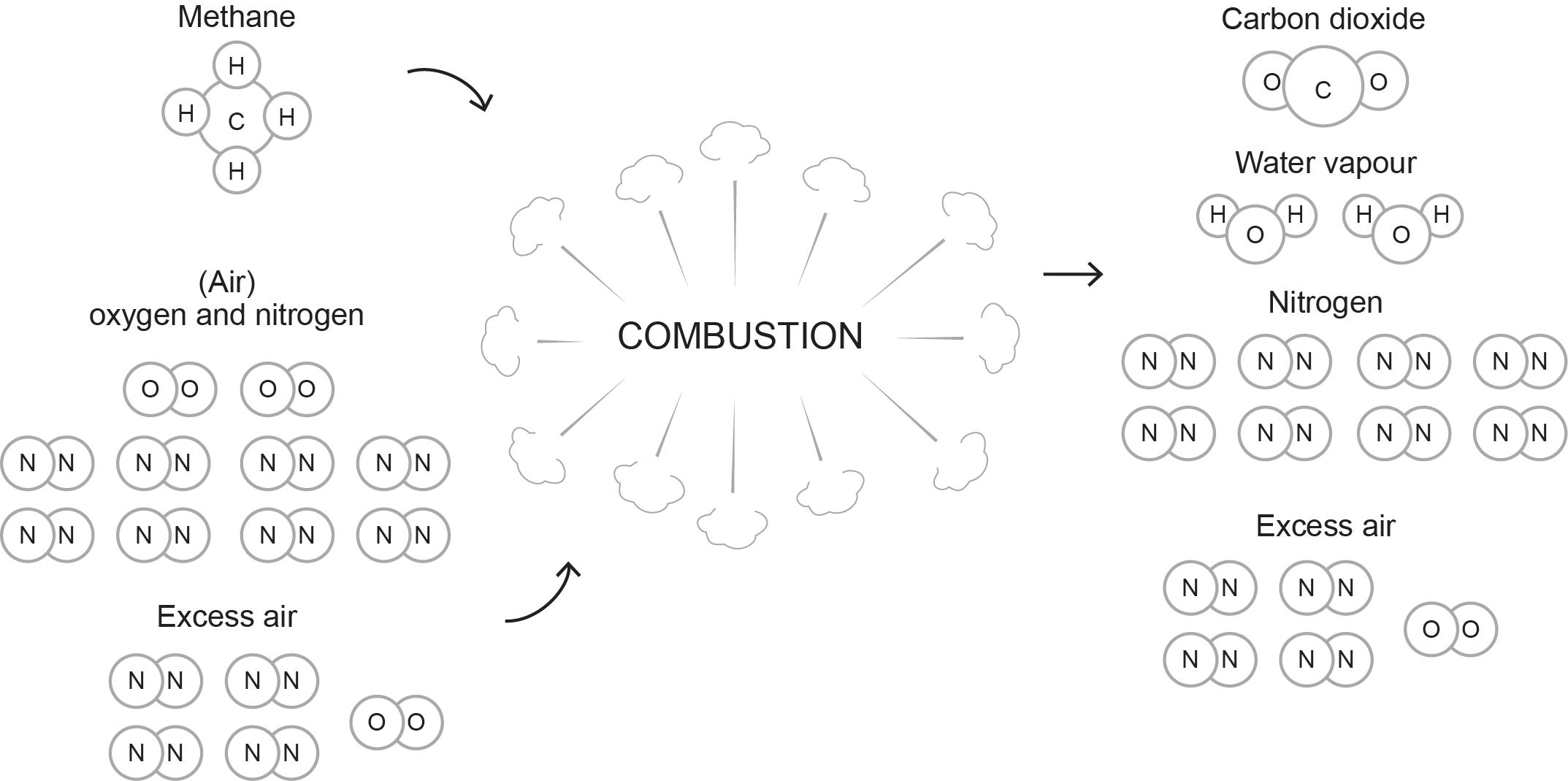
The complete combustion formula for propane is expressed as follows:
[latex]\color{blue}{\text{C}_3\text{H}_8+5\text{O}_2+20\text{N}_2+\text{Excess Air}\rightarrow3\text{CO}_2+4\text{H}_2\text{O}+20\text{N}_2+\text{Excess Air}+\text{Heat}}[/latex]
 Self-Test A-3.1: The Chemistry of Combustion
Self-Test A-3.1: The Chemistry of Combustion
Complete Self-Test A-3.1 and check your answers.
If you are using a printed copy, please find Self-Test A-3.1 and Answer Key at the end of this section. If you prefer, you can scan the QR code with your digital device to go directly to the interactive Self-Test.

References
Skilled Trades BC. (2021). Book 1: Fuel gas systems, Heating and cooling systems. Plumber apprenticeship program level 2 book 1 (Harmonized). Crown Publications: King’s Printer for British Columbia.
Trades Training BC. (2021). A-3: Air supply for gas appliances. In: Plumber Apprenticeship Program: Level 2. Industry Training Authority, BC.
Media Attributions
All figures are used with permission from Skilled Trades BC (2021) unless otherwise noted.
- Figure 1 Fire tetrahedron (adapted) by Gustavb, via Wikimedia Commons, is in the public domain.
The rapid oxidation of fuel, accompanied by heat or heat and light. Combustion is essential for gas appliances to operate properly. (Section A-3.1)
A compound made of hydrogen and carbon atoms. Most fuel gases, like natural gas, propane, and butane, are hydrocarbons. (Section A-3.1)
When fuel burns with just the right (theoretical or mathematically exact) amount of oxygen, leaving only carbon dioxide (CO₂) and water (H₂O) as products. It’s a perfectly balanced reaction, but it's hard to achieve in real life because it's difficult to get the exact mix of oxygen and fuel. (Section A-3.1)
The ideal burning process where there is enough oxygen to burn all the fuel completely, producing only carbon dioxide (CO₂), water vapor (H₂O), and heat as byproducts. (Section A-3.1)
Occurs when there is insufficient oxygen for the fuel to burn completely, producing hazardous byproducts like carbon monoxide (CO), soot, and aldehydes. (Section A-3.1)

